Aseptic Action is in Plastics
As aseptic packaging makes its move into plastics, packaging systems suppliers are positioning themselves to serve this evolving market. High-pressure processing development is well under way.
By Judy Rice, Contributing Writer
Table of Contents
Joint R&D Venture for Dairy
Mott's: an Aseptic Leader
High-Pressure Processing for Aseptics
"The phemomenal market interest in packaging in PET (polyethylene terephthalate) bottles will fuel the market growth for aseptic packaging."
That's a prediction offered by a Packaging Strategies/International Aseptic Technology research report released in January 1999. Consumers are PET-friendly for the obvious reasons — the packaging is shatter-proof, lightweight, recloseable, recyclable and can be produced in almost unlimited sizes and shapes. Its potential reaches way beyond the established applications in carbonated beverages and refrigerated products.

Packaging systems suppliers are positioning themselves to serve this evolving market. Even Tetra Pak, long known as an international pioneer in the brick-pack-style aseptic packaging arena, has made licensing arrangements to market aseptic PET technology manufactured by Rossi Catelli. Other notables include but are not limited to Remy, Rommelag, Bosch, Hassia, Engineered Products and Systems (EPSI), Tuchenhagen and Stork. Some of these systems utilize preformed PET bottles, while others blow-mold, fill and seal in one operation.
As noted in the Packaging Strategies research report, a number of U.S. food/beverage companies already have ventured into the aseptic — no refrigeration required — PET arena. Some of the more notable companies include General Mills, whose Squeeze-Its are packaged on a Rommelag system; Mott's, whose 64-oz PET aseptic bottles of juice are packaged on an EPSI system; and Quaker Oats, which has isotonic beverages in aseptic PET packaged on a Procomac system. And several other food and beverage manufacturers are gearing up to produce aseptic products in PET containers.
Joint R&D Venture for Dairy
In a related development, the Dutch company Stork, which has U.S. headquarters in Gainesville, GA, has just launched a joint aseptic R&D venture with the Dairy Farmers of America Inc. (DFA) (Kansas City, MO). Dubbed ASEP-TECH USA, the venture's pilot plant is being built in Springfield, MO, near the DFA Technology Center. DFA is financing the construction and will provide technical staff to man the facility. Stork will supply aseptic processing and packaging equipment, including a linear filler capable of handling a variety of container structures and sizes.
The ASEP-TECH pilot plant is expected to be fully operational by the year 2000, and will be available to provide development and test run work for both DFA and non-DFA members. While dairy beverages in plastic containers will be a major focus, non-dairy and particulate products and various container structures also will be researched there.
The Packaging Strategies 142-page aseptic update report is available for purchase for $2,995. For more information, contact Packaging Strategies, Inc. at 610-436-4220.
Mott's: an Aseptic Leader
In 1993, Mott's Aspers, PA, plant was the company's first facility to install an aseptic plastic bottle juice packaging line. Using 64-oz, patented "Easy Grip" blow-molded plastic bottles, Mott's is marketing a variety of juices, including apple, grape, grapefruit, orange, cranberry juice cocktail and tomato in the aseptic containers.
"This packaging provides convenient, cost-effective beverage solutions for our foodservice customers and will help them sucessfully manage their bar and beverage businesses," notes Tom Malkiewicz, director-marketing, foodservice and alternate channels for Mott's. The company, a subsidiary of London-based Cadbury Schweppes plc, and based in Stamford, CT, recently added 86-oz "Easy Grip" twin-pack bottles to its packaging repertoire. This new size responds primarily to the needs of clubstores and foodservice outlets.
Mott's also markets Mr. & Mrs. T Bloody Mary Mix products in aseptic plastic packaging. Non-refrigerated shelf-life for both the juices and Bloody Mary mixes is approximately one year.
High-Pressure Processing for Aseptics
Development for aseptics is underway at the Illinois Institute of Technology's National Center for Food Safety and Technology (Summit-Argo, IL). At the heart of the Center are two high-pressure processing lines for extended-shelf-life and shelf-stable product research and development. High-pressure processing inactivates microorganisms in food products by accelerating the destruction of microbial enzymes.
The two high-pressure processing systems that the Center is operating utilize technologies supplied by Flow International and ABB Autoclave Systems. The two companies recently pooled their high-pressure technologies to form Flow Pressure (Kent, WA).
Dr. Chuck Sizer is the director of the Center. Sizer reports that NCFS&T is available to work with food companies interested in exploring the potential of high-pressure processing and other food safety technologies.
IIT associate professor of food processing, Dr. V. M. Balasubramaniam (affectionately known as Dr. Bala for short) told Packaging Network: "One of the high-pressure systems housed at the Center is a pilot-scale batch system for processing of products that are pre-packaged. The pre-packaged products are placed into a pressure vessel and can be pressurized at up to 130,000 psi over a temperature range of 40-170°F. Batch processing can accommodate both liquid and solid products, but it limits the producer to pressure-compatible containers with at least one elastic interface."
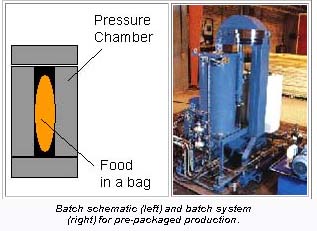
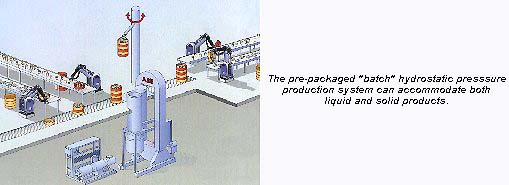
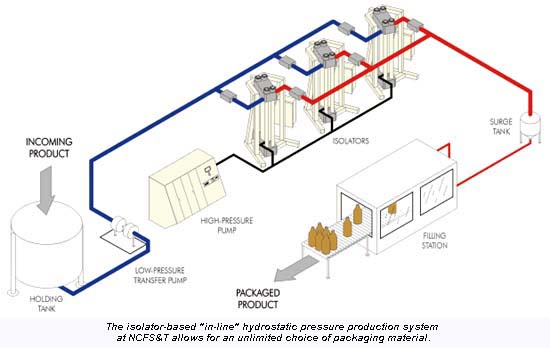
The second system at NCFS&T, installed this past summer, is a semi-continuous system for processing juices and other pumpable foods. "With this system, product is pumped into and out of the pressure vessel. Special valves allow rapid filling and emptying of the vessel. The processing steps include aseptic filling, pressurizing, holding, depressurizing and unloading," explains Bala.
By using multiple isolators, a semi-continuous process can be attained. This in-line approach typically needs the use of clean or aseptic filling if the product is not refrigerated. The choice of packaging material for the in-line process is unlimited since filling is performed after pressure exposure. However, good barrier property containers are usually needed to protect the higher quality product typically used with UHP. In-line processing is limited to pumpable products.
Bala adds, "Currently, many higher-value products that are heat-sensitive can benefit from high-pressure technology. Initial commercial products are high-acid or refrigerated foods with extended shelf-life. High-pressure-processed jams and jellies are available in Japan. Fresh orange juice with extended shelf-life and processed with high pressure is sold in Europe."
And Bala reports that high-pressure-processed guacamole is the first commercially available product in the United States. The manufacturer is Avomex Inc. of Keller, Texas. Bala said that NCFS&T is currently conducting bacterial spore inactivation studies to assess the effectiveness of high-pressure processing technology for producing shelf-stable products."
Center director Sizer summarizes, "The newest high-pressure system we have is a prototype capable of pasteurizing juice products, which then can be aseptically packaged in a range of containers — cans, plastic bottles, pouches. But even refrigerated products that are not categorized as ‘conventionally aseptic' can benefit from this technology because their shelf-life, organoleptic properties and microbiological integrity all are enhanced by high-pressure processing. And they aren't subjected to the potentially damaging effects of thermal treatment."
"Right now," Sizer continues, "what high-pressure gives you is a high-quality, high-cost product. But over time, cost of the technology should decrease. I think we have some realistic hopes for high-pressure's future."
For details about the National Center for Food Safety & Technology services and capabilities, contact Dr. Chuck Sizer at 708-563-1576. The Center has FDA personnel on-site to offer expertise and guidance to food companies and can offer assistance in putting companies on the fast track for petition clearances.
For more information:
Packaging Strategies, West Chester, PA, Tel: 610-436-4220.
National Center for Food Safety & Technology, Summit-Argo, IL, Tel: 708-563-1576.
Flow Pressure, Kent, WA, Tel: 253-850-3500.
